Glaucoma is described as a chronic, progressive, optic neuropathy which may be caused by a group of ocular conditions leading to damage to the retinal ganglion cells and optic nerve with corresponding visual field defects.Intraocular pressure(IOP) has been suggested to be the only modifiable risk factor.The main aim of treatment is to lower the IOP which slowsthe progression of the disease. The various options to reduce IOP includetopical or systemic medications, laser or surgical methods. The first line of therapy is topical medication. Laser trabeculoplasty has been used to lower IOP in open-angle glaucoma and ocular hypertensive patients.1 Angle closure glaucoma necessitates a laser peripheral iridotomy. The most common guarded filtration procedure is still the trabeculectomy which was described in the 1960s by Cairns. The usual indications of trabeculectomy include uncontrolled glaucoma despite maximum medical therapy, in instances of poor compliance with medical treatment or when the eye has advanced glaucoma requiring lower target pressure. However, the incidence of complications in trabeculectomy is significantly more than cataract surgery alone.2 Various reports have shown that phacoemulsification by itself reduces IOP.3 For a patient, who does not need a large IOP reduction phacoemulsification may suffice.
Since the start of the century, various new minimally invasive techniques or devices have been developed which have been described as the Minimally / Micro invasive glaucoma surgery (MIGS). Most of these are to be used in conjunction with phacoemulsification which helps lower the IOP further thereby reducing the dependence on medication. The greatest advantage is that the conjunctiva is spared which could be used to plan future trabeculectomy. Performing phacoemulsification with or without MIGS does not preclude a future trabeculectomy.
To call a procedure as MIGS has 5 main requirements.
- Ab-interno microincision: This spares the conjunctiva, prevents significant external scarring, allows future filtration surgery.
- Minimal disruption of normal anatomy: MIGS enhancesthe normal outflow.
- Effective in both lowering IOP and reducing medication use. There should at least be a 20% reduction in IOP from baseline.
- High safety profile: The incidence of complications needs to be significantly less than that of trabeculectomy.
- Rapid recovery like phacoemulsification.
The common indications and contraindications of MIGS have been described in the Table.
| Indications | Relative Contraindications |
|---|---|
| Mild to moderate glaucoma | Anomalies of the anterior chamber angle |
| Open angle glaucoma – Mainly Primary open angle glaucoma, Pigmentary glaucoma, Pseudoexfoliation glaucoma | Angle closure glaucoma |
| Noncompliant or develop adverse reactions to medication | Extensively scarred conjunctiva |
| Uncontrolled IOP with topical medication or laser trabeculoplasty |
Classification
- Increasing trabecular outflow by bypassing the trabecular meshwork (TM)
- Stent placement
- iStent(Glaukos, Laguna Hills, CA)
- iStent Inject(Glaukos, Laguna Hills, CA)
- Hydrus (Ivantis Inc., Irvine, CA)
- Tissue Excision
- Gonioscopy-Assisted Transluminal Trabeculotomy (GATT)
- Trabectome(NeoMedix Corp., San Juan Capistrano, CA)
- Kahook Dual Blade Goniotomy (KDB, New World Medical Inc., Rancho Cucamonga, CA)
- TRAB 360 system (Sight Sciences, Menlo Park, CA)
- Excimer Laser Trabeculostomy(AIDA, Glautec AG, Nurnberg, Germany)
- Stent placement
- Enhancing aqueous outflow through Schlemm’s canal
- VISCO360 device (Sight Sciences, Menlo Park, CA)
- Ab Interno Canaloplasty (ABiC) using the iTrack microcatheter system (EllexiScience, Fremont, CA)
- Drainage through a sub-conjunctival pathway
- XEN Gel Stent (Allergan Inc., Irvine, CA)
- Drainage through supra-choroidal pathways
- CyPass Micro-Stent (Alcon, Fort Worth, Texas)
- Reducing aqueous production
- Endocyclophotocoagulation(BVI, Little Silver, NJ)

Increasing trabecular outflow by bypassing the trabecular meshwork
Stent placement
- iStent
FDA Approval: 2012 for use in conjunction with phacoemulsification
Heparin-coated, non-ferromagnetic titanium stent
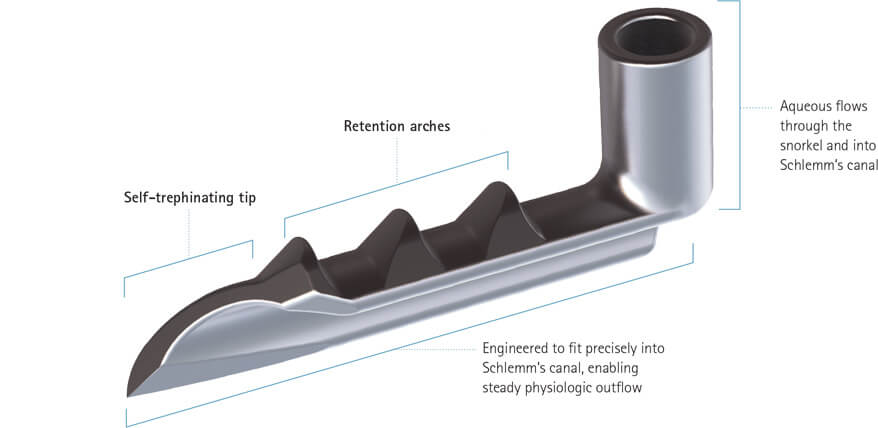
Snorkel shaped
Measures 0.3mm height x 1mm long
Central lumen: 120 μm
The device is implanted into the schlemm’s canal thereby directly connecting the anterior chamber to it. It has three retention arches to ensure that the device remains in situ.4(Figure 1)
After phacoemulsification and IOL implantation, the pupil is constricted using intracameral pilocarpine. The anterior chamber is filled with a cohesive viscoelastic agent to maintain the space. The injector is inserted through the clear corneal incision and then, under direct visualization using a gonioscope, the iStents are implanted through the trabecular meshwork into Schlemm’s canal.
Figure 1. iStent (www.glaukos.com)
- iStent Inject
- FDA Approval: June 2018 for use in conjunction with phacoemulsification
- Heparin-coated, non-ferromagnetic titanium stent
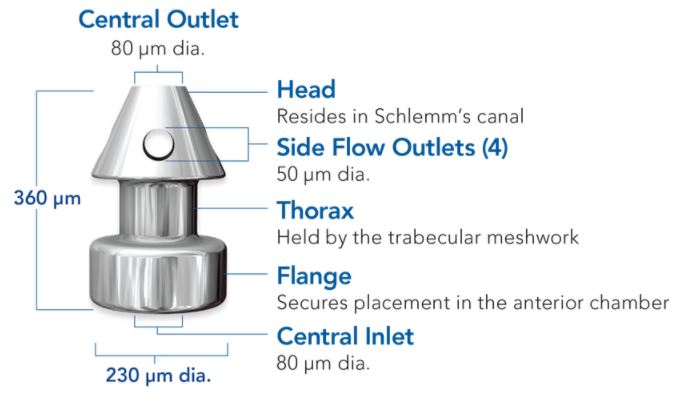
- 360 μm high x 230 μm diameter
The device consists of 3 parts (Figure 2)
- Head – lies in the schlemm’s canal
- Thorax – passes through the trabecular meshwork
- Flange – stays in the anterior chamber
- Central lumen: 80 μm
- The implantation is similar to that of an iStent.
Figure 2. iStent Inject (https://www.glaukos.com/healthcare-professionals/istent-inject/)
- Hydrus microstent
FDA Approval: August 2018 for use in conjunction with phacoemulsification
8mm long, 290 μm diameter
The device has three windows and an inlet. The windows pass into the schlemm’s canal and the inlet lies in the anterior chamber.
Material: Nitinol, a flexible, biocompatible titanium and nickle alloy
The device occupies 90 degrees of the trabecular meshwork.
In addition to bypassing the trabecular meshwork, this also acts as a scaffold to maintain the patency and dilates approximately three clock hours of the schlemm’s canal.5 (Figure 3)
Similar to an iStent implant, Hydrus implantation is performed via a clear corneal incision. After completing phacoemulsification, the microscope and patient head are adjusted to allow for a clear view of the nasal angle structures using a direct goniolens. The anterior chamber is filled with a cohesive viscoelastic agent to maintain the space. The Hydrus microstent is then introduced into the anterior chamber. The tip of the cannula is used to incise the trabecular meshwork following which the microstent is passed into the schlemm’s canal till its inlet. The inlet stays inside the anterior chamber. After positioning, the injector is withdrawn and viscoelastics is removed.
Figure 3. Hydrus microstent (https://www.ivantisinc.com/)

Tissue Excision
Gonioscopy-Assisted Transluminal Trabeculotomy (GATT)
Initially described by Grover et al in 2014.
This may be combined with phacoemulsification or performed as a stand-alone procedure.
The procedure involves creating a 360-degree ab interno trabeculotomy. A temporal clear corneal incision is made, and the anterior chamber is filled with cohesive viscoelastics. Under visualization using a direct gonioscopy lens like the Swan Jacob lens, a goniotomy is made in the opposite trabecular meshwork i.e. on the nasal side. A 5-0 prolene suture is then slowly threaded into the schlemm’s canal. Once it has been passed through the entire circumference, the distal end of the suture is grasped, and the proximal end is pulled out. This shears the trabecular meshwork thereby creating a 360-degree trabeculotomy. Alternatively, an iTrack microcatheter (Ellex iScience, Fremont, CA) can be used instead of the suture.6
Trabectome
Invented by George Baerveldt
FDA approved in April 2004
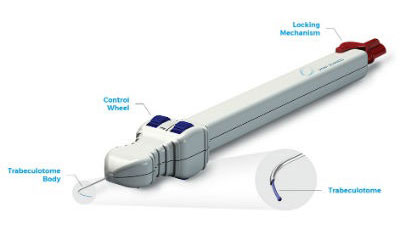
The device consists of a 3-stage footpedal to control the aspiration and ablation, a handle with an infusion sleeve, aspiration port, and a coupling for the ablation unit at the tip. The tip is bent 90° leading to a triangular footplate configuration. The footplate has a moderately sharp point to facilitate penetration of the meshwork into schlemm’s canal and is coated with a smooth insulating material to help it glide along within the canal and protect the canal’s outer wall and adjacent tissues from thermal or mechanical injury. The hook design serves to feed trabecular and juxtacanalicular tissues into the ablative device as the instrument tip is advanced through angle tissues. It utilizes a bipolar 550 kHz electrode, with adjustable power, to ablate the TM. The plasma that is generated has a highly confined heat dissipation with minimal thermal transfer to the outer wall.7 (Figure 4)
A beveled temporal clear corneal incision is made. The anterior chamber was filled with cohesive viscoelastics. The head and microscope are tilted appropriately and a surgical goniolens is placed on the cornea to visualize the nasal angle. The Trabectome hand piece is inserted through the clear corneal incision. The probe is then used to ablate between 90-120° of the nasal trabecular meshwork and inner wall of schlemm’s canal. The viscoelastic material is then washed off.
Figure 4. Trabectome
Kahook Dual Blade Goniotomy
Launched in the United States in 2015. (Figure 5)
This may be combined with phacoemulsification or performed as a stand-alone procedure.
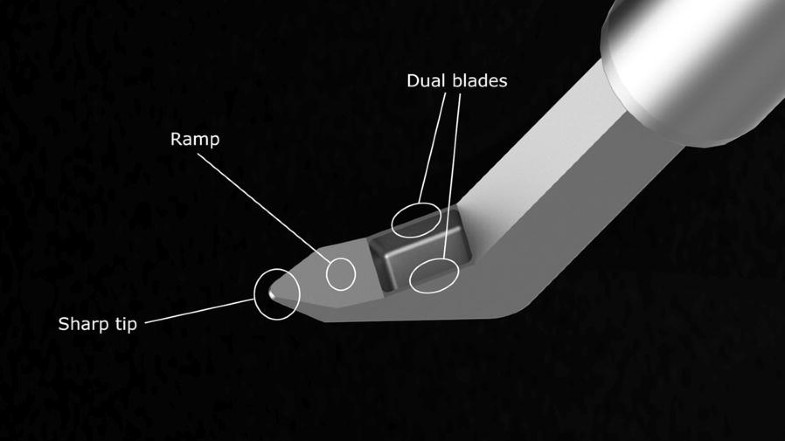
The Kahook dual blade is inserted into the eye through a clear corneal incision. It has a sharp tip which pierces the trabecular meshwork and enters into the schlemm’s canal. The ramp lifts and stretches the trabecular meshwork. As the device is moved circumferentially along the canal, the dual blades which are parallel to each other excises a parallel strip of the trabecular meshwork thereby exposing the outer wall of schlemm’s canal. The strip of trabecular meshwork can be removed with intraocular forceps or by aspiration.8
Figure 5. Kahook Dual Blade. (Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-Month Outcomes of Goniotomy Performed Using the Kahook Dual Blade Combined with Cataract Surgery in Eyes with Medically Treated Glaucoma. Adv Ther. 2018;35(9):1460-1469.)
TRAB 360 system
Received Health Canada approval in December 2016.
This device facilitates performance of an ab interno trabeculotomy like the GATT. (Figure 6) The tip of the hand piece is used to pierce the trabecular meshwork following which the microcatheter is then advanced from the tip of the device to cannulate 180 degrees of the schlemm’s canal. Then the device is withdrawn out. The microcatheter which was inside the schlemm’s canal tears the trabecular meshwork as it comes out. The device is then rotated, and the procedure is repeated for the remaining 180 degrees of the angle.9
Figure 6. TRAB 360 system
Excimer Laser Trabeculostomy
ExTra Laser System (MLase AG, Germering, Germany; ExTra ELT)
Received a Conformité Européenne (CE) mark in 2014
Laser energy is used to photoablate the trabecular meshwork tissue. A 308-nm xenon-chloride (XeCl) excimer laser is used.
1.2–1.3 mJ with a duration of 80 ns.
200-μm trabeculostomy openings are made through the trabecular meshwork and the inner wall of schlemm’s canal (Figure 7)
Estimated treatment depth of 20 μm
Under topical anaesthesia, a small paracentesis is made. The anterior chamber is filled with cohesive viscoelastics. Then, a fiber-optic probe is passed across the anterior chamber and placed in contact with the trabecular meshwork to punch a hole into it and the inner wall of schlemm’s canal. This creates a direct communication with the schlemm’s canal and aqueous is able to pass into it without much resistance.10
Figure 7. Excimer Laser Trabeculostomy.
(Berlin MS. Laser treatment for outflow obstruction. In: Stamper RL, editor. Diagnosis and Therapy of the Glaucomas. 8th Ed. Missouri: Mosby Elsevier; 2009. p. 453.)

Enhancing aqueous outflow through schlemm’s canal
- Visco 360 Viscosurgical System
Received Conformité Européenne (CE) Mark Approval in 2016 and Health Canada approval in 2017.
This can be used as a stand-alone procedure or combined with phacoemulsification.
This the allows for catheterization and viscodilation of schlemm’s canal. It works similar to TRAB 360 system. The tip of the hand piece is used to pierce the trabecular meshwork following which the microcatheter is then advanced from the tip to cannulate 180 degrees of the schlemm’s canal. Then, a predetermined amount of viscoelastics is injected into schlemm’s canal. This procedure is then repeated for the other 180 degree. The procedure allows for dilation of schlemm’s canal and collapsed collector channels without tissue destruction of the natural drainage system.11
The OMNI® Surgical System combined the TRAB 360 system with the Visco 360 Viscosurgical System thereby it targets all 3 areas where resistance is offered for the flow of aqueous – Trabecular meshwork, schlemm’s canal and the collector channels. (Figure 8)
U.S. FDA approval was obtained in January 2018.
Figure 8. OMNI® Surgical System (https://omnisurgical.com)

Ab Interno Canaloplasty (ABiC)
Described by Dr. Mark Gallardo
Similar to a GATT using a microcatheter, a nasal goniotomy is made. The microcatheter is then advanced into the schlemm’s canal circumferentially. As the catheter is slowly withdrawn, viscodilation is performed here. The injected viscoelastics causes dilation of the schlemm’s canal which leads to small ruptures in its inner wall and the adjacent trabecular meshwork. This creates a direct communication between the anterior chamber and the schlemm’s canal resulting in drainage without much resistance.12
Drainage through a sub-conjunctival pathway
XEN gel stent
FDA approved in November 2016
6 mm long, soft, flexible tube
Composed of a gelatin derived from porcine dermis and then cross-linked with glutaraldehyde
Inner lumen diameter: 45 μm
Outer diameter: 150 μm
The XEN gel stent is loaded onto an injector with a 27-gauge needle.The injector is inserted through a clear corneal incision into the anterior chamber. The tip of the injector is used to penetrate the trabecular meshwork and the sclera to reach the subconjunctival space from ab interno. The slider of the injector is then advanced to deploy the stent which creates a direct communication between the anterior chamber and the subconjunctival space. Mitomycin C may be injected subconjunctivally before or after stent placement to reduce subconjunctival fibrosis.13 (Figure 9)
Figure 9. XEN gel stent
- PRESERFLO® MicroShunt (Santen, Osaka, Japan)
Formerly known as the InnFocus MicroShunt
This was the first MIGS device to undergo a head-to-head comparative trial versus trabeculectomy in USA and Europe.
MicroShunt received a CE Mark in 2012 and is commercially available in Europe.
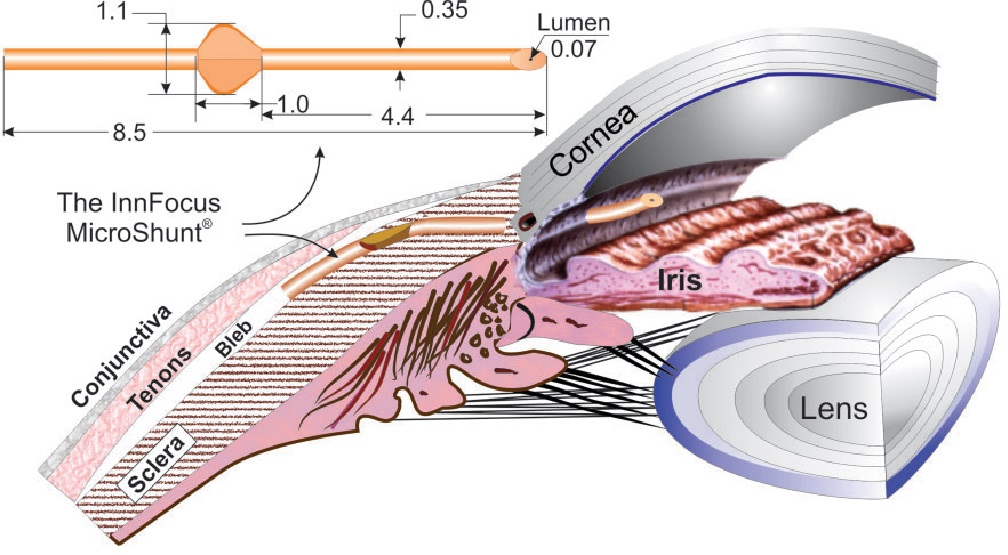
Ab Externo device – The tube is inserted through a scleral flap into the anterior chamber. Additional use of antimetabolites like Mitomycin-C or 5-FU ensures long-term survival of the bleb.
The MicroShunt has an 8.5-mm long styrene-block-isobutylene-block-styrene tube with an outer diameter of 350 μm and a lumen diameter of 70 μm. This luminal diameter was found to prevent hypotony and reduce clogging. It has 2 external fins to prevent migration and to seal the incision. (Figure 10)
The device shunts aqueous from the anterior chamber to the sub-conjunctival/sub-Tenon space.14
Figure 10.
Drainage through supra-choroidal pathways
The suprachoroidal space has a negative hydrostatic pressure relative to the anterior chamber. This pressure differential serves to continuously draw aqueous from the anterior chamber to the suprachoroidal space through these devices.
Ab interno approach
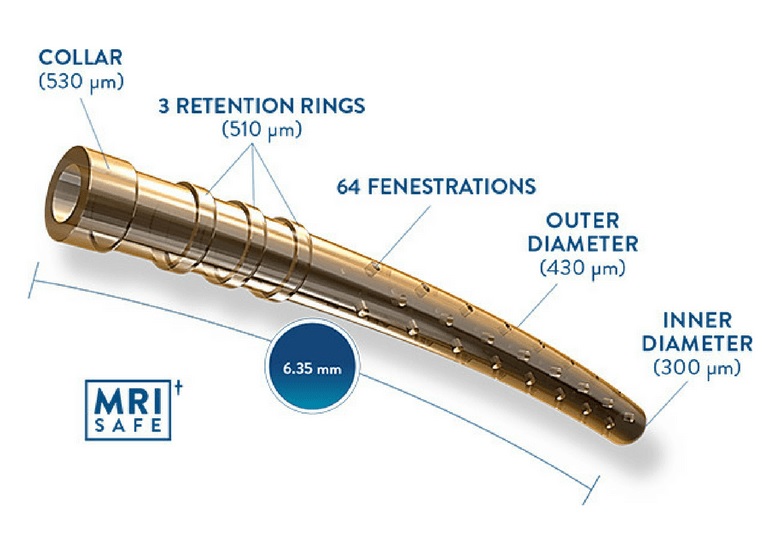
CyPass Micro-Stent
FDA approved in July 2016
CyPass was withdrawn from the market in August 2018 when it was noted that there was a significant decrease in corneal endothelial cell counts in patients undergoing CyPass with phacoemulsification compared to phacoemulsification alone. Nevertheless, it was the only device approved for supra-choroidal implant.
Made of polymide
It creates a controlled cyclodialysis cleft with subsequent drainage into the suprachoroidal space thereby increasing the uveo-scleral outflow.15 6.35 mm long
Outer diameter: 430 μm
Inner diameter: 300 μm
64 fenestrations each 76 μm in diameter is seen along the length of the device
- The proximal end has three retention rings to aid in stability. (Figure 11)
Figure 11. CyPass Micro-Stent (http://new-glaucoma-treatments.com/cypass-glaucoma-micro-stent-two-year-results/)
Other devices pending FDA approval for supra-choroidal implantation18
Ab interno approach
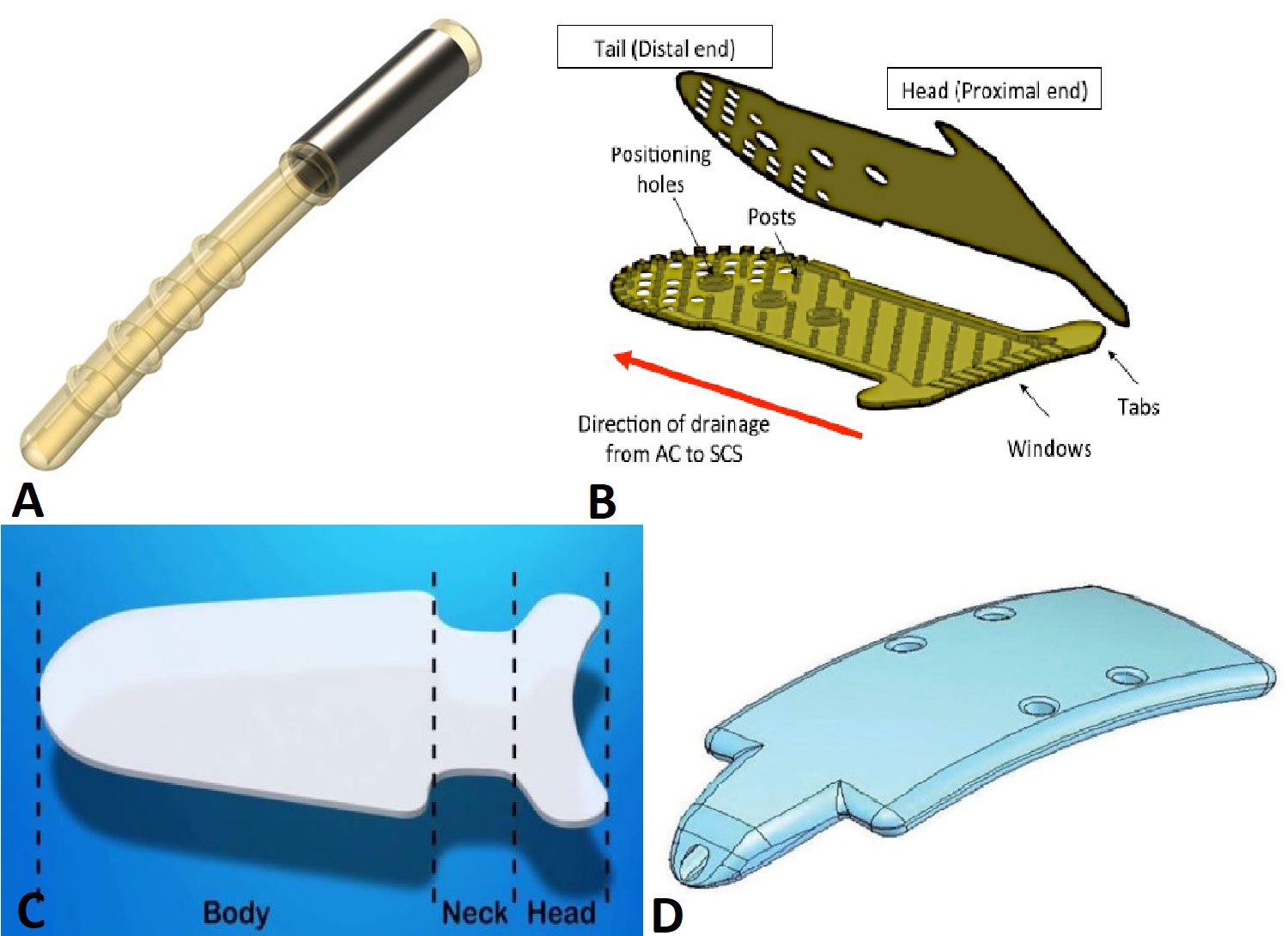
iStent Supra (Glaukos Corporation, Laguna Hills, CA, USA)
It is a 4mm tube with a hole at each end.
Made of Polyethersulfone (PES) and titanium (Figure 12 A)
The insertion is similar to that of the CyPass Micro-Stent
Ab externo approach
SOLX gold shunt (SOLX Inc., Waltham, MA, USA)
CE-marked: 2005
5.2 mm long x 3.5 mm wide x 68 μm thick.
It is made of biocompatible, inert, 99.9% pure gold and includes an internal passageway for aqueous fluid transfer. The proximal end or head of the device has channels which allow aqueous flow into the device from the anterior chamber. The shape of the proximal end is concave to reduce the possibility of contact with the iris or corneal endothelial surface. The distal or tail end that terminates in the suprachoroidal space has openings that allow aqueous flow through that end. Larger reinforced openings aligned along the centerline are designed to help position the device. A protective, sterile inserter is supplied with each device to aid with handling and insertion of the implant.16, 17(Figure 12 B)
SOLX’s DeepLight Glaucoma Treatment System combines its Titanium Sapphire Laser and the Gold Shunt. A 790 nm Titanium Sapphire Laser is used similar to trabeculoplasty to provide deeper trabecular penetration to about 200 μm without causing thermal, coagulative or biodestructive damage. It emits near-infrared light (790 nm) in pulses lasting 5-10 microseconds. The Gold Micro-Shunt implant contains multiple micro-channels some of which are initially held in reserve. These reserve channels can be opened later using the DeepLight laser.
STARflo Glaucoma Implant (iSTAR Medical SA, Wavre, Belgium)
CE marked: 2012
It is made of a proprietary flexible, micro-porous medical grade silicone called STAR Biomaterial, which is designed to optimize tissue integration and reduce fibrosis.
It is a porous implant and has 3 parts – a head, neck and body. (Figure 12 C)
8 mm long x 5 mm (3 mm at the neck) wide x 275 μm thick.
A conjunctival flap followed by a rectangular 6–7 mm x 3 mm superficial scleral flap of 50% depth is created. The head is implanted in the anterior chamber at the anterior aspect of the scleral flap. A full thickness scleral incision is made at the posterior edge of the flap. The body of the device is then inserted below the sclera into the suprachoroidal space. The scleral and conjunctival flaps are then sutured tightly to avoid leakage of aqueous and bleb formation.
Aquashunt (OPKO Health Inc, Miami, FL, USA)
It is made of polypropylene and has a single 250 μm lumen.
4mm wide x 10mm long x 0.75mm thick. (Figure 12 D)
Figure 12. Supra-choroidal implants. A. iStent Supra, B. SOLX gold shunt, C. STARflo Glaucoma Implant, D. Aquashunt
(Surgical Innovations in Glaucoma. Editors: Samples, John R., Ahmed, Iqbal Ike K. Springer 2014
Tanito M, Chihara E. Safety and effectiveness of gold glaucoma micro shunt for reducing intraocular pressure in Japanese patients with open angle glaucoma. Jpn J Ophthalmol. 2017;61(5):388-394.
Kammer JA, Mundy KM. Suprachoroidal devices in glaucoma surgery. Middle East Afr J Ophthalmol. 2015;22(1):45-52.)
Reducing aqueous production
Endocyclophotocoagulation (ECP)
Diode laser of 810 nm is used for photocoagulation of the ciliary processes.18
It works by creating inflammation, thrombosis and destruction of the ciliary epithelium thereby reducing aqueous secretion.
An endoscopic probe is connected to a video monitor for visualization and is attached to the laser unit (BVI, Little Silver, NJ) which is foot pedal controlled.
The probe is inserted into the anterior chamber through a clear corneal incision. Laser power is titrated to achieve both blanching and contraction of the ciliary processes. Around 200-360 degrees of the angle is treated in a single sitting. (Figure 13)
Figure 13. (https://crstoday.com/wp-content/themes/crst/assets/article-images/2016-09/Goldman_fg1.jpg)

In conclusion, MIGS have added a long list of options to the glaucoma surgeon in mild to moderate glaucomas. Moreover, MIGS can be easily added to routine phacoemulsification and if IOP is not sufficiently controlled, a future trabeculectomy can be planned. However, in advanced glaucomas, trabeculectomy or glaucoma drainage devices still works best due to greater IOP reduction.
References :
- Garg A, Gazzard G. Selective laser trabeculoplasty: past, present, and future [published correction appears in Eye (Lond). 2020 Aug;34(8):1487]. Eye (Lond). 2018;32(5):863-876.
- Tham CC, Kwong YY, Baig N, Leung DY, Li FC, Lam DS. Phacoemulsification versus trabeculectomy in medically uncontrolled chronic angle-closure glaucoma without cataract. Ophthalmology. 2013 Jan;120(1):62-7.
- Sengupta S, Venkatesh R, Krishnamurthy P, et al. Intraocular Pressure Reduction after Phacoemulsification versus Manual Small-Incision Cataract Surgery: A Randomized Controlled Trial. Ophthalmology. 2016;123(8):1695-1703.
- Samuelson T, Katz LJ, Wells JM, Duh Y, Giamporcaro J. Randomized Evaluation of the Trabecular Micro-Bypass Stent with Phacoemulsification in Patients with Glaucoma and Cataract. Ophthalmology 2011; 118: 459-467.
- Samuelson TW, Chang DF, Marquis R, et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract: The HORIZON Study. Ophthalmology. 2019;126(1):29-37.
- Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855-861.
- Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the Trabectome for treatment of open-angle glaucoma [published correction appears in Ophthalmology. 2005 Sep;112(9):1540]. Ophthalmology. 2005;112(6):962-967.
- Greenwood M, Seibold L, Radcliffe N, Dorairaj S, Aref A, RomanJ, Lazcano-Gomez G, Darlington J, Abdullah S, Jasek M, Bahjri K, Berdahl J. Goniotomy with a single-use dual blade: Short term results. J Cataract Refract Surg 2017; 43: 1197-1201.
- Sarkisian S, Mathews B, Ding K, Patel A, Nicek Z. 360o ab-interno trabeculotomy in refractory open-angle glaucoma. Clinical Ophthalmology 2019; 13: 161-168.
- Durr GM, Töteberg-Harms M, Lewis R, Fea A, Marolo P, Ahmed IIK. Current review of Excimer laser Trabeculostomy. Eye Vis (Lond). 2020;7:24.
- Ondrejka S and Korber N. 360o ab-interno schlemm’s canal viscodilation in primary open angle glaucoma. Clinical Ophthalmology 2019; 13: 1235-1246.
- Gallardo M, Supnet R, Ahmed II. Viscodilation of schlemm’s Canal for the Reduction of IOP Via an Ab-interno Approach. Clinical Ophthalmology 2018; 12: 2149-2155.
- Grover DS, Flynn WJ, Bashford KP, et al. Performance and Safety of a New Ab Interno Gelatin Stent in Refractory Glaucoma at 12 Months. Am J Ophthalmol. 2017;183:25-36.
- Sadruddin O, Pinchuk L, Angeles R, Palmberg P. Ab externo implantation of the MicroShunt, a poly (styrene-block-isobutylene-block-styrene) surgical device for the treatment of primary open-angle glaucoma: a review. Eye Vis (Lond). 2019;6:36.
- Hoeh H, Vold SD, Ahmed IK, et al. Initial Clinical Experience With the CyPass Micro-Stent: Safety and Surgical Outcomes of a Novel Supraciliary Microstent. J Glaucoma. 2016;25(1):106-112.
- Tanito M, Chihara E. Safety and effectiveness of gold glaucoma micro shunt for reducing intraocular pressure in Japanese patients with open angle glaucoma. Jpn J Ophthalmol. 2017;61(5):388-394. doi:10.1007/s10384-017-0520-2
- Gigon A, Shaarawy T. The Suprachoroidal Route in Glaucoma Surgery. J Curr Glaucoma Pract. 2016;10(1):13-20. doi:10.5005/jp-journals-10008-1197
- Uram M. Endoscopic Cyclophotocoagulation in Glaucoma Management. Current Opin Ophthalmol 1995; 6(2): 19-29.
By,
Dr Annamalai Odayappan
Glaucoma Consultant,
Aravind Eye Hospital,
Pondicherry
